The Cellular Secrets of LLLT: ATP, Mitochondria, and Healing Unleashed
1. Introduction: Unveiling the Power of Light at the Cellular Level
The intersection of photonic energy and cellular biology represents one of the most fascinating frontiers in modern regenerative medicine. Low-Level Laser Therapy (LLLT), also known as photobiomodulation (PBM), harnesses the therapeutic potential of specific wavelengths of light to stimulate cellular repair mechanisms and accelerate healing processes. This non-invasive therapeutic approach operates at the subcellular level, targeting mitochondria—the powerhouses of our cells—to enhance adenosine triphosphate (ATP) production and initiate a cascade of beneficial biological responses. The emergence of LLLT as a clinically validated treatment modality has revolutionized our understanding of how light energy can be transformed into biochemical energy, ultimately promoting tissue regeneration, reducing inflammation, and alleviating pain. As healthcare providers and researchers continue to unlock the mechanisms behind photobiomodulation, we are witnessing a paradigm shift in how we approach cellular healing and tissue repair.
2. The Science Behind LLLT: Light Meets Biology
Understanding the fundamental science behind LLLT requires examining the intricate relationship between photonic energy and cellular biochemistry. This section explores the core mechanisms that make light therapy a powerful tool for healing.
2.1 What Is Low-Level Laser Therapy (LLLT) and How Does It Work?
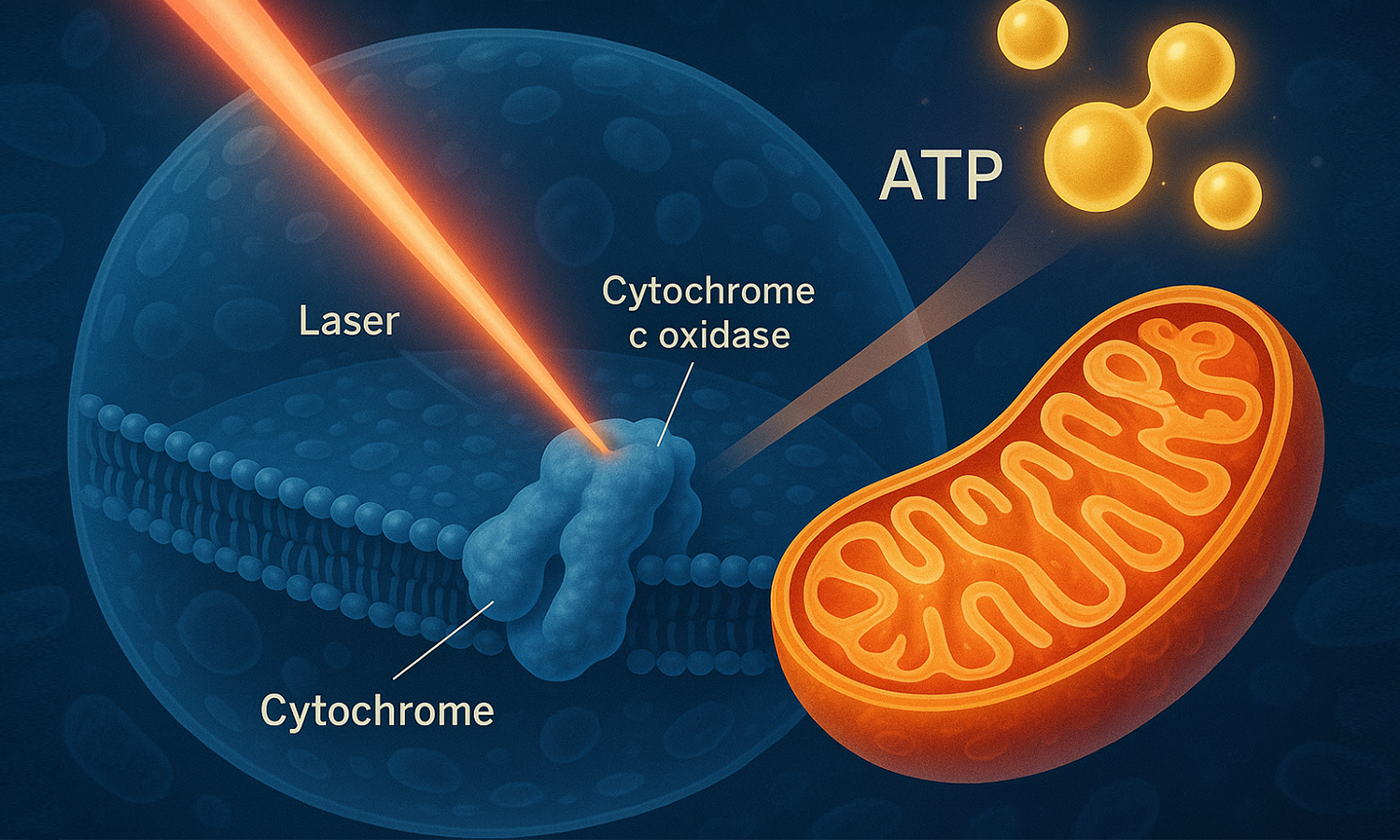
Low-Level Laser Therapy (LLLT) is a clinical application of laser light to the body with aims to modulate tissue recovery and repair, decrease inflammation and/or reduce pain, utilizing low-power lasers or light-emitting diodes (LEDs) applied to the surface of the body. Unlike high-power lasers used in surgical procedures that cut or destroy tissue, LLLT operates at therapeutic power densities typically ranging from 1-100 mW/cm². The therapy employs specific wavelengths, primarily in the red (660-670 nm) and near-infrared (810-980 nm) spectrum, which correspond to the absorption peaks of cytochrome c oxidase, the primary photoacceptor in mitochondria. These wavelengths penetrate tissue effectively while avoiding harmful thermal effects, making LLLT a safe and non-invasive treatment option.
2.2 Photobiomodulation: The Cellular Conversation
Photobiomodulation represents the sophisticated dialogue between light energy and cellular components. PBM involves the use of red or near-infrared light at low power densities to produce a beneficial effect on cells or tissues. This process initiates when photons are absorbed by chromophores, particularly cytochrome c oxidase (Complex IV) in the mitochondrial electron transport chain. The absorbed photonic energy triggers conformational changes in the enzyme, leading to enhanced electron transport efficiency and improved cellular respiration. This photochemical process, distinct from thermal effects, represents a fundamental mechanism of cellular energy enhancement. The specificity of wavelength absorption ensures that therapeutic effects are targeted and controlled, optimizing cellular function without causing tissue damage.
2.3 The Role of Mitochondria: The Cell's Powerhouse
Mitochondria serve as the primary target for LLLT intervention, functioning as cellular powerhouses responsible for energy production. PBM treatment causes NO to dissociate from Complex IV (cytochrome c oxidase, CCO), causing the complex's activity to increase. This allows the flux of electrons, the pumping of protons, and the synthesis of ATP to increase, thereby boosting cellular energy levels. Under normal physiological conditions, nitric oxide (NO) can bind to cytochrome c oxidase, particularly under hypoxic or inflammatory conditions, reducing its activity and consequently decreasing ATP production. LLLT effectively reverses this inhibition by promoting NO dissociation, restoring optimal mitochondrial function. This mechanism is particularly relevant in damaged or stressed tissues where mitochondrial dysfunction contributes to impaired healing and persistent inflammation.
3. ATP: The Energy Currency of Healing
The enhancement of ATP production through LLLT represents a fundamental mechanism underlying the therapy's healing effects. This section examines how increased ATP availability translates into accelerated tissue repair and recovery.
3.1 What Is ATP and Why It Matters
Adenosine triphosphate (ATP) serves as the universal energy currency of cellular processes, providing the necessary energy for virtually all biological functions. ATP consists of adenosine bound to three phosphate groups, with the high-energy phosphate bonds storing and releasing energy through hydrolysis. In the context of healing, ATP is essential for protein synthesis, DNA replication, membrane transport, muscle contraction, and immune cell activation. Cellular energy depletion often accompanies tissue injury, inflammation, and disease states, creating a metabolic bottleneck that impairs healing processes. Understanding ATP's central role in cellular metabolism explains why enhancing its production through LLLT can have profound therapeutic effects across diverse medical conditions.
3.2 How LLLT Stimulates ATP Production for Faster Healing
The action of PBM includes increasing ATP production, activating ATP-dependent ion pumps, and regulating calcium flux. LLLT enhances ATP production through multiple interconnected mechanisms. The primary pathway involves the photostimulation of cytochrome c oxidase, which increases electron transport chain efficiency and drives enhanced oxidative phosphorylation. This process occurs within the mitochondrial cristae, where the increased proton gradient generated by improved electron transport directly correlates with ATP synthase activity. Additionally, LLLT influences calcium homeostasis by modulating mitochondrial calcium uptake and release, which further optimizes ATP production. The therapy also affects cellular membrane potential, creating favorable conditions for ATP synthesis while simultaneously reducing cellular energy expenditure through improved ion pump efficiency.
3.3 Benefits of Higher ATP Levels in Tissue Repair
Elevated ATP levels following LLLT treatment provide cells with the necessary energy substrate for accelerated tissue repair processes. Enhanced ATP availability drives increased protein synthesis, particularly collagen production, which is essential for wound healing and tissue regeneration. Higher energy levels also support enhanced cellular proliferation, allowing for more rapid replacement of damaged cells and tissue structures. ATP-dependent processes such as active transport mechanisms become more efficient, improving nutrient delivery and waste removal from healing tissues. Additionally, increased ATP levels support enhanced immune cell function, promoting more effective inflammatory resolution and tissue remodeling. The energy boost also enables cells to maintain better stress resistance and antioxidant defense mechanisms, creating optimal conditions for sustained healing and recovery.
4. Cellular Benefits Beyond ATP
While ATP enhancement forms the foundation of LLLT's therapeutic effects, the therapy produces numerous additional cellular benefits that contribute to its clinical efficacy. This section explores these complementary mechanisms.

4.1 Anti-Inflammatory Effects at the Cellular Level
LLLT is hypothesized to modulate cellular metabolism, tissue microenvironment(s) and to decrease inflammation while posing few adverse risks. The anti-inflammatory effects of LLLT operate through multiple cellular pathways beyond simple ATP enhancement. Photobiomodulation influences the nuclear factor-kappa B (NF-κB) signaling pathway, a master regulator of inflammatory responses, by modulating its activation and translocation to the nucleus. This results in decreased production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6). Simultaneously, LLLT promotes the expression of anti-inflammatory mediators, including interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), creating a favorable environment for tissue healing and repair.
4.2 Enhanced Blood Flow and Oxygenation with LLLT
LLLT promotes significant improvements in microcirculation and tissue oxygenation through direct effects on vascular endothelial cells and smooth muscle. The therapy stimulates nitric oxide production in endothelial cells, leading to vasodilation and improved blood flow to treated tissues. This enhanced perfusion increases oxygen and nutrient delivery while facilitating more efficient removal of metabolic waste products. LLLT also influences angiogenesis, the formation of new blood vessels, by upregulating vascular endothelial growth factor (VEGF) expression and promoting endothelial cell proliferation. The improved oxygenation supports enhanced cellular respiration and ATP production, creating a positive feedback loop that amplifies the therapeutic effects. Additionally, better circulation reduces tissue hypoxia, a common contributor to chronic pain and delayed healing.
4.3 How LLLT Helps Nerve Regeneration and Reduces Pain Naturally
The neurological benefits of LLLT extend beyond simple pain reduction to include actual nerve regeneration and repair. Photobiomodulation enhances Schwann cell proliferation and myelination, critical processes for peripheral nerve regeneration. The therapy increases the production of neurotrophic factors, including nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), which promote axonal growth and survival. LLLT also modulates pain signaling pathways by influencing sodium and potassium channel activity in neurons, reducing abnormal pain signal transmission. The therapy's effects on inflammation contribute to pain reduction by decreasing inflammatory mediators that sensitize nociceptors. Additionally, LLLT promotes the release of endogenous opioids and influences serotonin levels, providing natural pain relief through multiple neurotransmitter pathways.
5. Clinical Implications and Applications
The diverse cellular mechanisms of LLLT translate into broad clinical applications across multiple medical specialties. This section examines specific therapeutic applications and their underlying mechanisms.
5.1 Musculoskeletal Pain and Injury Recovery
LLLT demonstrates remarkable efficacy in treating musculoskeletal conditions by addressing multiple pathophysiological mechanisms simultaneously. In tendinopathies, the therapy promotes collagen synthesis and fiber organization while reducing inflammatory infiltrates and pain mediators. For muscle injuries, LLLT enhances satellite cell activation and myoblast proliferation, accelerating muscle regeneration and reducing recovery time. The therapy's anti-inflammatory effects help resolve acute and chronic inflammatory states in joints, tendons, and muscles. Enhanced ATP production supports the energy-intensive processes of tissue repair, while improved circulation delivers essential nutrients and removes metabolic waste products. Clinical studies have shown significant improvements in pain scores, functional capacity, and healing times across various musculoskeletal conditions, including tendinitis, muscle strains, and joint pain.
5.2 Treating Neuropathy and Nerve Damage with Low-Level Laser Therapy
Neuropathic conditions respond particularly well to LLLT due to the therapy's direct effects on nerve tissue regeneration and pain modulation. In diabetic neuropathy, LLLT addresses both the metabolic dysfunction and inflammatory components of the condition. The therapy improves nerve conduction velocity by enhancing myelination and reducing inflammatory damage to nerve fibers. LLLT stimulates the production of neurotrophic factors essential for nerve survival and regeneration, while simultaneously reducing oxidative stress that contributes to nerve damage. The therapy's analgesic effects provide relief from neuropathic pain by modulating pain signal transmission and reducing peripheral sensitization. Clinical applications include treatment of diabetic peripheral neuropathy, post-herpetic neuralgia, and various forms of peripheral nerve injuries, with studies showing improvements in pain levels, sensory function, and quality of life.
5.3 Inflammation Control in Chronic Conditions
Chronic inflammatory conditions benefit from LLLT's ability to modulate immune responses and resolve persistent inflammation. The therapy influences macrophage polarization, promoting the transition from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype, which is crucial for inflammation resolution. LLLT reduces the production of inflammatory prostaglandins and leukotrienes while enhancing the synthesis of specialized pro-resolving mediators (SPMs) that actively promote inflammation resolution. The therapy also influences T-cell function, promoting regulatory T-cell activity and reducing harmful inflammatory responses. Applications include treatment of rheumatoid arthritis, osteoarthritis, inflammatory bowel conditions, and chronic wounds. The therapy's ability to address inflammation at the cellular level makes it particularly valuable for conditions where conventional anti-inflammatory treatments may be insufficient or associated with significant side effects.
5.4 Post-Surgical and Orthopedic Rehabilitation
LLLT plays an increasingly important role in post-surgical recovery and orthopedic rehabilitation by accelerating healing and reducing complications. The therapy enhances wound healing by promoting fibroblast proliferation and collagen synthesis, leading to stronger and more organized scar tissue formation. LLLT reduces post-operative pain and swelling, potentially decreasing the need for pain medications and their associated side effects. The therapy's anti-inflammatory effects help prevent excessive scar tissue formation and promote optimal tissue remodeling. Enhanced circulation supports better oxygen and nutrient delivery to healing tissues, while improved lymphatic drainage reduces edema and promotes toxin removal. Clinical applications include post-operative wound care, fracture healing, joint replacement recovery, and sports injury rehabilitation, with studies demonstrating reduced healing times, improved functional outcomes, and decreased complication rates.
6. Safety, Dosage, and Protocol Considerations
Optimal therapeutic outcomes with LLLT require careful consideration of treatment parameters and safety protocols. This section addresses the critical factors that influence treatment efficacy and safety.
6.1 Choosing the Right Wavelengths and Power Settings for Effective LLLT
The selection of appropriate wavelengths and power settings is crucial for maximizing therapeutic benefits while ensuring patient safety. Red light wavelengths (660-670 nm) provide excellent absorption by cytochrome c oxidase and penetrate superficial tissues effectively, making them ideal for wound healing and surface treatments. Near-infrared wavelengths (810-980 nm) offer deeper tissue penetration, making them suitable for treating deeper structures such as muscles, joints, and nerves. Power densities should typically range from 1-100 mW/cm² to achieve photobiomodulation without thermal effects. The biphasic dose response characteristic of LLLT means that both insufficient and excessive doses can reduce therapeutic efficacy. Optimal dosing depends on tissue type, condition severity, and treatment depth requirements. Professional-grade devices with calibrated output ensure consistent and reproducible treatment parameters.
6.2 Treatment Frequency and Duration: Finding the Sweet Spot
Treatment frequency and duration require careful optimization based on the specific condition, patient characteristics, and desired outcomes. Acute conditions typically respond well to daily treatments initially, with frequency gradually reduced as healing progresses. Chronic conditions may require longer treatment courses with 2-3 sessions per week over several weeks or months. Treatment duration per session typically ranges from 5-20 minutes, depending on the area size and power density used. The cumulative dose concept is important, as therapeutic effects are influenced by both individual session parameters and overall treatment course design. Overtreatment can lead to dose saturation and reduced efficacy, while undertreatment may fail to achieve desired outcomes. Regular assessment and protocol adjustment based on patient response ensure optimal therapeutic results while minimizing treatment time and cost.
7. The Future of LLLT in Regenerative Medicine
As our understanding of photobiomodulation deepens, LLLT is emerging as a powerful tool in regenerative medicine. Future innovations include AI-driven personalization, advanced wavelength combinations, and compact home-use devices that make treatment more accessible and precise. LLLT is also being explored in combination with stem cell therapy, platelet-rich plasma, and other regenerative methods to enhance therapeutic outcomes. Targeted nanoparticle systems activated by specific wavelengths offer exciting potential for highly precise cellular interventions. Clinical trials are expanding LLLT's scope into areas like neurodegenerative disease, cardiovascular care, and personalized aesthetic medicine. The integration of biomarker monitoring and real-time adjustment tools may soon optimize outcomes and minimize patient variability. With both technology and research accelerating, LLLT is poised to play a central role in the next generation of personalized, non-invasive regenerative therapies.
8. Real-World Outcomes: What Patients and Practitioners Say
Clinical use of LLLT reveals consistent improvements in recovery, pain relief, and patient satisfaction. Practitioners across fields report faster healing, reduced treatment times, and better outcomes compared to conventional therapies alone. Physical therapists see quicker recovery in sports injuries, with fewer re-injuries. Pain specialists note significant relief in chronic conditions, while dental professionals observe reduced post-op pain and faster healing. Patients appreciate the therapy’s non-invasive nature and minimal side effects, especially as an alternative to medications or surgery. Many report improvements within a few sessions, with steady progress over time. Its comfort, convenience, and lack of downtime make LLLT a practical choice for a wide range of individuals, including those unable to pursue more invasive treatments. These real-world results, from clinic to home, reinforce LLLT’s growing reputation as a safe, effective, and accessible tool in modern care.

9. Conclusion: Healing Unleashed at the Speed of Light
LLLT harnesses the power of light to stimulate mitochondrial activity and ATP production, triggering a cascade of cellular responses that accelerate tissue repair, reduce inflammation, and relieve pain. Its influence on metabolism, immune modulation, and regeneration makes it a versatile and effective therapeutic tool. Backed by growing scientific evidence and a strong safety profile, LLLT stands out as a non-invasive option in modern healthcare. As photobiomodulation research progresses, and technology advances—especially in precision delivery and home-use devices—LLLT is set to become increasingly central in regenerative medicine. The future of LLLT lies not only in its solo applications but also in its integration with other therapies like stem cells and PRP, forming powerful, multi-layered healing protocols. By continuing to unlock the cellular secrets of light, we move closer to truly personalized, light-speed healing solutions.
10. FAQs
Q1. Can shining a light on my skin really boost cell energy? How does that work?
Yes! LLLT stimulates mitochondria—the cell’s energy factories—using specific wavelengths (e.g., 810nm) to increase ATP production. This "light charge" jumpstarts healing from within.
Q2. What makes LLLT different from regular heat or infrared lamps?
Unlike general heat lamps, LLLT uses targeted, coherent laser light that penetrates deeper and activates cellular photoreceptors—not just warming the tissue. The result? Precise biological responses, not just comfort.
Q3. Is it possible to overdo LLLT? Can too much light slow healing?
Surprisingly, yes. LLLT follows a biphasic dose response—too little light won’t work, and too much may reduce effectiveness. That’s why treatment parameters matter.
Q4. Can I use home-use LLLT devices instead of going to a clinic?
Home devices are growing in popularity. While clinical units are more powerful, well-designed home models offer convenience for maintenance therapy and milder conditions—especially with AI guidance emerging.
Q5. Does LLLT only work on muscles and joints, or can it help nerves too?
LLLT isn’t just for sore muscles—it also supports nerve regeneration by boosting cellular metabolism and reducing neuroinflammation. Many neuropathy patients report great results.
Q6. Could LLLT be part of futuristic medicine, like stem cells or nanotherapy?
Absolutely. Research is exploring LLLT in combo with stem cell therapy, PRP, and even light-activated nanoparticles. It’s becoming a core tool in next-gen regenerative medicine.
11. References
Photobiomodulation in human muscle tissue: an advantage in sports performance?
Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP.
Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring

